How can climate change affect water itself?
Climate change can indirectly affect water quality and aquatic life by causing physical or chemical changes in the water. For example, higher water temperatures will reduce oxygen concentrations in the water. Climate change can also contribute to changes in concentrations of micropollutants, salt or nutrients in water. These are referred to as physio-chemical changes. All such physio-chemical changes affect the quality of surface water.
Would you like to know more about the various properties of the water system and how they can be affected by climate change? The page on How vulnerable is a water system to climate change? may be of interest, as will the interactive knowledge document Urban Water Quality, Climate and Adaptation (pdf, 13 MB).
Tool helps to improve water quality
The various properties of a water system together determine its water quality. Altering the value of such characteristics enables you to influence or “control” the water quality. Are you interested in improving the water quality of a water system? The Water Quality Roadmap and the Urban Water Quality, Climate and Adaptation Tool will be helpful in this respect. The Roadmap mainly helps you to identify potential measures that affect the properties. The Tool indicates the limiting values of properties relating to functional uses. For example, a water system must have a temperature of 25 to 32 degrees Celsius in order to serve as swimming water.
Hypoxia
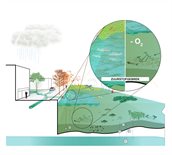
As a result of climate change, hypoxia (low oxygen levels) will occur more frequently in surface water. Hypoxia can be caused by heat and by severe precipitation:
- Heat: Oxygen concentrations in water largely depend on the water temperature. If the water temperature is high, the oxygen concentration will decrease. One of the reasons is that water organisms use more oxygen in warmer water. In a heatwave, the water can even turn anoxic. The latter occurs particularly in shallow bodies of water whose bed is covered with a layer of organic matter, e.g. originating from fallen leaves. As this water will heat up more rapidly, fish and other wildlife species there will consume more oxygen. In addition, the decomposition of organic matter (mineralisation) requires a great deal of oxygen.
- Severe precipitation: Torrential rain may affect the oxygen concentration in surface water, particularly during hot summers. In such situations, if severe downpours are also causing more frequent sewer overflows, the demand for oxygen will increase, thus potentially resulting in anoxia. Fish and other small water creatures cannot survive in anoxic water. Furthermore, hypoxia will affect the composition of species.
Gooré Bi E, Monette F, Gasperi J. Analysis of the influence of rainfall variables on urban effluents concentrations and fluxes in wet weather. Journal of Hydrology. 2015; 523:3 20-32.
Sand-Jensen KAJ, Pedersen NL, Søndergaard M. Bacterial metabolism in small temperate streams under contemporary and future climates. Freshwater Biology. 2007; 52 (12): 2340-53.
Moss B, Kosten S, Meerhoff M, Battarbee RW, Jeppesen E, Mazzeo N, et al. Allied attack: climate change and eutrophication. Inland Waters. 2011;1(2):101-5.
Kosten S, Schep S, van Weeren BJ. Een frisse blik op warmer water: Over de invloed van klimaatverandering op de aquatische ecologie en hoe je de negatieve effecten kunt tegengaan. Amersfoort: STOWA; 2011.
More (micro) pollutants and toxins
Climate change may result in more micropollutants and toxins ending up in surface water. In the event of severe precipitation, more micropollutants will flush into surface water, either directly through runoff or indirectly through leaching via groundwater. The combination of warm weather and persistent drought may also have a harmful impact: for example, in former industrial or agricultural locations, the waterbed may release toxins. Click on the questions below for a more detailed explanation.
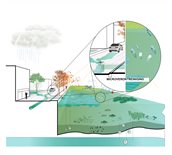
Particularly after a prolonged period of drought, intense downpours may cause micropollutants to end up in surface water. Such micropollutants could be tyre dust, copper from car brakes, microplastics, pesticides, veterinary pharmaceuticals or oil residue. Micropollutants are harmful to life in and around surface water. For many locations, hardly any data is available on the concentration of micropollutants in surface water. In most cases, the tests conducted by water management bodies only cover a few micropollutants, as such tests are quite costly.
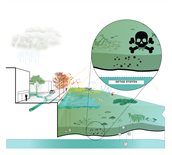
Under certain conditions, the soil underneath bodies of water in former industrial locations can release toxins. The same goes for bodies of water in farmland. This risk is highest in hot weather and in water that has been stagnant for a prolonged period of time due to persistent drought. In warm temperatures, toxins or toxic residues (aka degradation products) are released more quickly. If they end up in the water, such substances will seriously harm life in and around the surface water. This risk is going to increase in the future, as we will see increasingly frequent warm and dry periods, alternating with torrential rain.
Sun N, Yearsley J, Baptiste M, Cao Q, Lettenmaier DP, Nijssen B. A spatially distributed model for assessment of the effects of changing land use and climate on urban stream quality. Hydrological Processes. 2016; 30(25): 4779-98.
Mahbub P, Goonetilleke A, Ayoko GA, Egodawatta P. Effects of climate change on the wash-off of volatile organic compounds from urban roads. Science of the Total Environment. 2011; 409(19): 3934-42.
De Nijs ACM, Driesprong A, den Hollander HA, de Poorter LRM, Verweij WHJ, Vonk JA, et al. Risico’s van toxische stoffen in de Nederlandse oppervlaktewateren Bilthoven: RIVM; 2008.
More salt (salinisation)
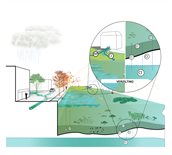
Climate change may raise the salt concentration (chloride) in surface water. This is referred to as salinisation. Salinisation can occur as a result of saltwater seepage and inlet:
- Salinisation caused by the influx of saline seawater via surface water or originating from external sources of salt (usually artificial, such as saline effluent) is referred to as external salinisation. For instance, in dry periods, we occasionally let in “foreign” water from the major rivers, even though such water may have quite a high salt content during summer.
- Salinisation as a result of saltwater seepage is referred to as internal salinisation. Internal salinisation occurs because of the increasing volumes of saltwater seepage flowing into freshwater bodies as a result of the rising sea level and the subsiding soil. This happens if the volume of freshwater is insufficient and seepage is turning the water saline.
Drought and heat can also cause salinisation of surface water. In surface water, a persistently high salt content as a result of salinisation may have a biological impact on the water.
S.A.H. Weisscher, R. van Ek, M. van der Kamp, T.M.J. te Winkel, dr. T.C. van Hateren, A.C. de Vos, dr. G. van Dijk, Dr. G.J. van Geest. Afwegingskader zoet-zout dynamiek. Kennisrapportage beschikbare kennis. Amersfoort: STOWA 2025-11.
KNMI. KNMI”14: Climate Change scenarios for the 21st Century – A Netherlands perspective. De Bilt: KNMI; 2014.
Kosten S, Schep S, van Weeren BJ. Een frisse blik op warmer water: Over de invloed van klimaatverandering op de aquatische ecologie en hoe je de negatieve effecten kunt tegengaan. Amersfoort: STOWA; 2011.
Too many nutrients due to eutrophication
As a result of climate change, phosphorus and nitrogen concentrations in surface water are increasing. Consequently, eutrophication is increasing: too many nutrients are ending up in the water. This may foster the growth of harmful blue-green algae, thus compromising water quality. Climate change entails two processes that raise the phosphorus and nitrogen content. The first process takes place in the surface water itself and is referred to as internal eutrophication. The second process is caused by sources outside the surface water and is known as external eutrophication. Click below for a detailed explanation of internal and external eutrophication.
Jeppesen E, Kronvang B, Meerhoff M, Søndergaard M, Hansen KM, Andersen HE, et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J Environ Qual. 2009; 38(5): 1930-41.
Kosten S, Schep S, van Weeren BJ. Een frisse blik op warmer water: Over de invloed van klimaatverandering op de aquatische ecologie en hoe je de negatieve effecten kunt tegengaan. Amersfoort: STOWA; 2011.
Boers PCM. Studying the phosphorus release from the Loosdrecht Lakes sediments, using a continuous flow system. Hydrobiological Bulletin. 1986; 20 (1): 51-60.
Genkai-Kato M, Carpenter SR. Eutrophication due to phosphorus recycling in relation to lake morphometry, temperature, and macrophytes. Ecology. 2005; 86 (1): 210-9.
Bouraoui F, Grizzetti B, Granlund K, Rekolainen S, Bidoglio G. Impact of Climate Change on the Water Cycle and Nutrient Losses in a Finnish Catchment. Climatic Change. 2004; 66 (1): 109-26.
Chang H. Water Quality Impacts of Climate and Land Use Changes in Southeastern Pennsylvania*. The Professional Geographer. 2004; 56 (2): 240-57.
Jeppesen E, Kronvang B, Olesen JE, Audet J, Søndergaard M, Hoffmann CC, et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia. 2011; 663 (1):1-21.
Paerl HW, Huisman J. Blooms Like It Hot. Science. 2008; 320 (5872): 57.
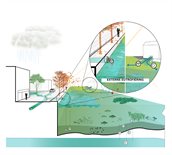
External eutrophication means that phosphorus and nitrogen concentrations in the water are increasing due to external sources. The main cause is changes in precipitation levels: on the one hand, increasing winter precipitation and torrential summer rain, and on the other, increasing summer drought, resulting in stagnant water. In addition, rain itself also contains nitrogen, so rain volumes play a part too.
Increase in nitrogen
The increase in nitrogen is mainly caused by increasing rainwater runoff and by sewer overflows onto surface water.
Increase in phosphorus
The increase in phosphorus concentrations is mainly caused by sewer overflows: sewage water contains a lot of phosphate, a common form of phosphorus. During severe precipitation, green rooftops and trees can also constitute a source of phosphorus. Another cause of external eutrophication is the inlet of foreign water. Water originating from rural farmland usually contains large concentrations of nutrients.
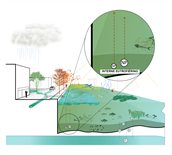
Internal eutrophication means that the increasing concentration of phosphorus and nitrogen in the water can be attributed to the aquatic system itself. This happens as follows: higher temperatures accelerate the decomposition of organic matter, both in the water and in the sediment at the bottom. The decomposition of organic matter releases phosphorus and nitrogen. Especially in shallow water, the temperature will rise rapidly in warm weather. In such cases, the sediment may release double the volume of phosphorus. The thicker the layer of sediment, the more phosphorus it may release. The top layer of the sediment could even turn anoxic, which would release additional phosphorus into the water. Furthermore, the temperature affects the pH values of the water and thus the rates of decomposition.
Stagnant water is conducive to the process
If water remains stagnant for a prolonged period of time due to drought, nitrogen and phosphorus concentrations will remain high. Flushing with flowing water may reduce the high nitrogen and phosphorus concentrations. In dry periods, inlet water may produce the same effect.